
- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Search
- Page Path
- HOME > Search
- Metabolic Risk/Epidemiology
- Novel Asian-Specific Visceral Adiposity Indices Are Associated with Chronic Kidney Disease in Korean Adults
- Jonghwa Jin, Hyein Woo, Youngeun Jang, Won-Ki Lee, Jung-Guk Kim, In-Kyu Lee, Keun-Gyu Park, Yeon-Kyung Choi
- Diabetes Metab J. 2023;47(3):426-436. Published online March 6, 2023
- DOI: https://doi.org/10.4093/dmj.2022.0099

- 2,491 View
- 128 Download
- 1 Web of Science
- 1 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
The Chinese visceral adiposity index (CVAI) and new visceral adiposity index (NVAI) are novel indices of visceral adiposity used to predict metabolic and cardiovascular diseases in Asian populations. However, the relationships of CVAI and NVAI with chronic kidney disease (CKD) have not been investigated. We aimed to characterize the relationships of CVAI and NVAI with the prevalence of CKD in Korean adults.
Methods
A total of 14,068 participants in the 7th Korea National Health and Nutrition Examination Survey (6,182 men and 7,886 women) were included. Receiver operating characteristic (ROC) analyses were employed to compare the associations between indices of adiposity and CKD, and a logistic regression model was used to characterize the relationships of CVAI and NVAI with CKD prevalence.
Results
The areas under the ROC curves for CVAI and NVAI were significantly larger than for the other indices, including the visceral adiposity index and lipid accumulation product, in both men and women (all P<0.001). In addition, high CVAI or NVAI was significantly associated with a high CKD prevalence in both men (odds ratio [OR], 2.14; 95% confidence interval [CI], 1.31 to 3.48 in CVAI and OR, 6.47; 95% CI, 2.91 to 14.38 in NVAI, P<0.05) and women (OR, 4.87; 95% CI, 1.85 to 12.79 in CVAI and OR, 3.03; 95% CI, 1.35 to 6.82 in NVAI, P<0.05); this association remained significant after adjustment for multiple confounding factors in men and women.
Conclusion
CVAI and NVAI are positively associated with CKD prevalence in a Korean population. CVAI and NVAI may be useful for the identification of CKD in Asian populations, including in Korea. -
Citations
Citations to this article as recorded by- Association between Chinese visceral adiposity index and risk of stroke incidence in middle-aged and elderly Chinese population: evidence from a large national cohort study
Zenglei Zhang, Lin Zhao, Yiting Lu, Xu Meng, Xianliang Zhou
Journal of Translational Medicine.2023;[Epub] CrossRef
- Association between Chinese visceral adiposity index and risk of stroke incidence in middle-aged and elderly Chinese population: evidence from a large national cohort study
- COVID-19
- Impact of Social Distancing Due to Coronavirus Disease 2019 on the Changes in Glycosylated Hemoglobin Level in People with Type 2 Diabetes Mellitus
- Sung-Don Park, Sung-Woo Kim, Jun Sung Moon, Yin Young Lee, Nan Hee Cho, Ji-Hyun Lee, Jae-Han Jeon, Yeon-Kyung Choi, Mi Kyung Kim, Keun-Gyu Park
- Diabetes Metab J. 2021;45(1):109-114. Published online December 4, 2020
- DOI: https://doi.org/10.4093/dmj.2020.0226
- 9,427 View
- 307 Download
- 23 Web of Science
- 24 Crossref
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub 
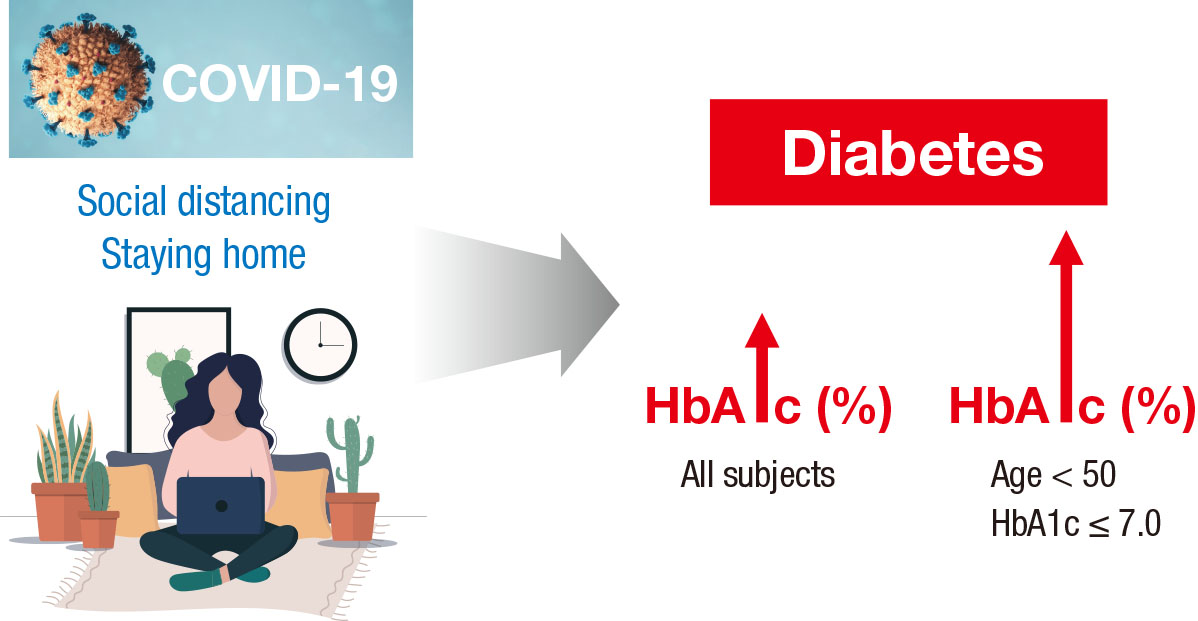
- This study investigated the impact of social distancing due to coronavirus disease 2019 (COVID-19) on glycemic control in people with type 2 diabetes mellitus (T2DM). We retrospectively analyzed the change in glycosylated hemoglobin level (ΔHbA1c) in people with T2DM who undertook social distancing because of COVID-19. We compared the ΔHbA1c between COVID-19 and non-COVID-19 cohorts that were enrolled at the same time of year. The ΔHbA1c of the COVID-19 cohort was significantly higher than that of two non-COVID-19 cohorts. Subgroup analysis according to age and baseline HbA1c level showed that social distancing significantly increased the mean HbA1c level of participants of <50 years. The ΔHbA1c of participants of <50 years and with HbA1c <7.0% in the COVID-19 cohort showed larger changes than other subgroups. In adjusted model, adjusted ΔHbA1c levels in the COVID-19 cohort remained significantly higher than those in the two other cohorts. Social distancing negatively impacts blood glucose control in people with T2DM, especially those who are younger and have good blood glucose control.
-
Citations
Citations to this article as recorded by- Impact of two COVID-19 lockdowns on HbA1c levels in patients with type 2 diabetes and associations with patient characteristics: a multicentre, observational cohort study over three years
Ingmar Schäfer, Daniel Tajdar, Laura Walther, Lasse Bittner, Dagmar Lühmann, Martin Scherer
Frontiers in Public Health.2024;[Epub] CrossRef - Influence of the COVID-19 pandemic on the achievement of guideline targets for HbA1c, blood pressure, and LDL cholesterol in people with diabetes in Japan
Shingo Kuwajima, Takahito Itoh, Tatsuya Sato, Shoya Ino, Satoru Shibata, Kouhei Ohno, Hiroyuki Hotta, Tomoaki Matsumoto, Hitoshi Ooiwa, Hirofumi Kubo, Takayuki Miki
Diabetology International.2024;[Epub] CrossRef - Socioeconomic status and the effect of prolonged pandemic confinement on anthropometric and glycaemic outcomes in adults with type 2 diabetes mellitus
Chandana Wijeweera, Ummul Muhfaza, Reginald V. Lord, Peter Petocz, Juliana Chen, Veronica Preda
Primary Care Diabetes.2024;[Epub] CrossRef - Physical and Mental Health Characteristics of Hospitalized COVID-19 Patients with and without Type 2 Diabetes Mellitus in Turkey
Abdulbari Bener, Murat Atmaca, Abdulla O. A. A. Al-Hamaq, Antonio Ventriglio
Brain Sciences.2024; 14(4): 377. CrossRef - Self-Care of Adults with Type 2 Diabetes During the COVID-19 Pandemic: A Qualitative Interpretive Description Study
Michela Luciani, Camilla Bigoni, Marta Canesi, Matteo Masotto, Diletta Fabrizi, Stefania Di Mauro, Davide Ausili
Clinical Nursing Research.2023; 32(1): 73. CrossRef - Changes in body weight and glycemic control in association with COVID-19 Shutdown among 23,000 adults with type 2 diabetes
Emily Panza, Kevin E. Kip, Kripa Venkatakrishnan, Oscar C. Marroquin, Rena R. Wing
Acta Diabetologica.2023; 60(6): 787. CrossRef - The Impact of a Lockdown for the COVID-19 Pandemic on Seasonal HbA1c Variation in Patients with Type 2 Diabetes
Yu-Cheng Cheng, Yu-Hsuan Li, Hsiu-Chen Liu, Chiann-Yi Hsu, Wan-Jen Chang, I-Te Lee, Chin-Li Lu
Life.2023; 13(3): 763. CrossRef - Changes in the mean incidence and variance of orthopedic diseases before and during the COVID-19 pandemic in Korea: a retrospective study
Joo-Hee Kim, Mi Jung Kwon, Hyo Geun Choi, Sang Jun Lee, Sangwon Hwang, Jaemin Lee, San-Hui Lee, Jung Woo Lee
BMC Musculoskeletal Disorders.2023;[Epub] CrossRef - Gender differences-based bioinformatics analysis to identify hub genes and key pathways in type 2 diabetes
Md Sojib Hossain, Subrina Islam Rupa, Md Sumon Sarkar, Md Al Amin, Mst Tania Khatun, Md Shamim, Md Zahidul Islam
Informatics in Medicine Unlocked.2023; 40: 101302. CrossRef - Retrospective Study on the Impact of COVID-19 Lockdown on Patients with Type 2 Diabetes in Northern Taiwan
Hsuan Huang, Hsiao-Ling Su, Chih-Hsung Huang, Yi-Hsin Lin
Diabetes, Metabolic Syndrome and Obesity.2023; Volume 16: 2539. CrossRef - Understanding impacts of COVID-19 restrictions on glycemic control for patients with diabetes in Japan
Kiyoko Uno-Eder, Noriko Satoh-Asahara, Manabu Hibiya, Kenji Uno, Takuya Uchino, Koji Morita, Toshio Ishikawa, Tetsuji Kaneko, Hajime Yamakage, Yuki Kitaoka, Tomohiro Sawa, Kazuhisa Tsukamoto, Tamio Teramoto
Journal of Diabetes & Metabolic Disorders.2023; 22(2): 1695. CrossRef - Impacts of the COVID-19 pandemic on unmet social needs, self-care, and outcomes among people with diabetes and poor glycemic control
Minal R. Patel, Guanghao Zhang, Cindy Leung, Peter X.K. Song, Michele Heisler, Hae Mi Choe, Roshanak Mehdipanah, Xu Shi, Kenneth Resnicow, Geila Rajaee, John D. Piette
Primary Care Diabetes.2022; 16(1): 57. CrossRef - Impact of the COVID-19 Pandemic on Glycemic Control and Blood Pressure Control in Patients with Diabetes in Japan
Keisuke Endo, Takayuki Miki, Takahito Itoh, Hirofumi Kubo, Ryosuke Ito, Kouhei Ohno, Hiroyuki Hotta, Nobuo Kato, Tomoaki Matsumoto, Aya Kitamura, Mai Tamayama, Takako Wataya, Ayaka Yamaya, Rei Ishikawa, Hitoshi Ooiwa
Internal Medicine.2022; 61(1): 37. CrossRef - The Effects of COVID-19 Lockdown on Glycaemic Control and Lipid Profile in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis
Omorogieva Ojo, Xiao-Hua Wang, Osarhumwese Osaretin Ojo, Edith Orjih, Nivedita Pavithran, Amanda Rodrigues Amorim Adegboye, Qian-Qian Feng, Paul McCrone
International Journal of Environmental Research and Public Health.2022; 19(3): 1095. CrossRef - Lifestyles Under Lockdown: A Scoping Review of International Studies on Type 2 Diabetes Self-Management Behaviors During COVID-19
Caroline Cummings, Kagnica Seng, Ryan Tweet, Julie Wagner
Frontiers in Clinical Diabetes and Healthcare.2022;[Epub] CrossRef - Substitution of telemedicine for clinic visit during the COVID‐19 pandemic of 2020: Comparison of telemedicine and clinic visit
Yukiko Onishi, Rieko Ichihashi, Yoko Yoshida, Tazu Tahara, Takako Kikuchi, Toshiko Kobori, Tetsuya Kubota, Masahiko Iwamoto, Shoko Hamano, Masato Kasuga
Journal of Diabetes Investigation.2022; 13(9): 1617. CrossRef - The impact of the COVID-19 pandemic on the management of patients with chronic diseases in Primary Health Care
Panagiotis Stachteas, Manolis Symvoulakis, Apostolos Tsapas, Emmanouil Smyrnakis
Population Medicine.2022; 4(August): 1. CrossRef - Effects of COVID-19 Pandemic and Lockdown on Monitoring and Treatment Balance of Finnish Coronary Heart Disease and Type 2 Diabetes Patients
Piia Lavikainen, Marja-Leena Lamidi, Teppo Repo, Laura Inglin, Janne Martikainen, Tiina Laatikainen
Clinical Epidemiology.2022; Volume 14: 1363. CrossRef - Impact of Social Distancing Due to Coronavirus Disease 2019 on the Changes in Glycosylated Hemoglobin Level in People with Type 2 Diabetes Mellitus (Diabetes Metab J 2021;45:109-14)
Junghyun Noh
Diabetes & Metabolism Journal.2021; 45(2): 275. CrossRef - Impact of Social Distancing Due to Coronavirus Disease 2019 on the Changes in Glycosylated Hemoglobin Level in People with Type 2 Diabetes Mellitus (Diabetes Metab J 2021;45:109-14)
Sung-Don Park, Sung-Woo Kim, Jun Sung Moon, Jae-Han Jeon, Mi Kyung Kim, Keun-Gyu Park
Diabetes & Metabolism Journal.2021; 45(2): 279. CrossRef - Glucose control in diabetes during home confinement for the first pandemic wave of COVID-19: a meta-analysis of observational studies
Giovanni Antonio Silverii, Chiara Delli Poggi, Ilaria Dicembrini, Matteo Monami, Edoardo Mannucci
Acta Diabetologica.2021; 58(12): 1603. CrossRef - The impact of COVID-19 pandemic on glycemic control in patients with diabetes mellitus in Turkey: a multi-center study from Kocaeli
Alev Selek, Emre Gezer, Eda Altun, Mehmet Sözen, Ömercan Topaloğlu, Damla Köksalan, Halil Demirkan, Dilek Karakaya, Berrin Cetinarslan, Zeynep Cantürk, Dilek Taymez
Journal of Diabetes & Metabolic Disorders.2021; 20(2): 1461. CrossRef - Effects of Social Distancing on Diabetes Management in Older Adults during COVID-19 Pandemic
Soo Myoung Shin, Tae Jung Oh, Sung Hee Choi, Hak Chul Jang
Diabetes & Metabolism Journal.2021; 45(5): 765. CrossRef - Year-Long Trend in Glycated Hemoglobin Levels in Patients with Type 2 Diabetes during the COVID-19 Pandemic
Jonghwa Jin, Seong Wook Lee, Won-Ki Lee, Jae-Han Jeon, Jung-Guk Kim, In-Kyu Lee, Yeon-Kyung Choi, Keun-Gyu Park
Endocrinology and Metabolism.2021; 36(5): 1142. CrossRef
- Impact of two COVID-19 lockdowns on HbA1c levels in patients with type 2 diabetes and associations with patient characteristics: a multicentre, observational cohort study over three years
- Covid-19
-
- The Clinical Characteristics and Outcomes of Patients with Moderate-to-Severe Coronavirus Disease 2019 Infection and Diabetes in Daegu, South Korea
- Mi Kyung Kim, Jae-Han Jeon, Sung-Woo Kim, Jun Sung Moon, Nan Hee Cho, Eugene Han, Ji Hong You, Ji Yeon Lee, Miri Hyun, Jae Seok Park, Yong Shik Kwon, Yeon-Kyung Choi, Ki Tae Kwon, Shin Yup Lee, Eon Ju Jeon, Jin-Woo Kim, Hyo-Lim Hong, Hyun Hee Kwon, Chi Young Jung, Yin Young Lee, Eunyeoung Ha, Seung Min Chung, Jian Hur, June Hong Ahn, Na-young Kim, Shin-Woo Kim, Hyun Ha Chang, Yong Hoon Lee, Jaehee Lee, Keun-Gyu Park, Hyun Ah Kim, Ji-Hyun Lee
- Diabetes Metab J. 2020;44(4):602-613. Published online August 12, 2020
- DOI: https://doi.org/10.4093/dmj.2020.0146

- 13,317 View
- 206 Download
- 67 Web of Science
- 74 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub Background Coronavirus disease 2019 (COVID-19) is a global pandemic that had affected more than eight million people worldwide by June 2020. Given the importance of the presence of diabetes mellitus (DM) for host immunity, we retrospectively evaluated the clinical characteristics and outcomes of moderate-to-severe COVID-19 in patients with diabetes.
Methods We conducted a multi-center observational study of 1,082 adult inpatients (aged ≥18 years) who were admitted to one of five university hospitals in Daegu because of the severity of their COVID-19-related disease. The demographic, laboratory, and radiologic findings, and the mortality, prevalence of severe disease, and duration of quarantine were compared between patients with and without DM. In addition, 1:1 propensity score (PS)-matching was conducted with the DM group.
Results Compared with the non-DM group (
n =847), patients with DM (n =235) were older, exhibited higher mortality, and required more intensive care. Even after PS-matching, patients with DM exhibited more severe disease, and DM remained a prognostic factor for higher mortality (hazard ratio, 2.40; 95% confidence interval, 1.38 to 4.15). Subgroup analysis revealed that the presence of DM was associated with higher mortality, especially in older people (≥70 years old). Prior use of a dipeptidyl peptidase-4 inhibitor or a renin-angiotensin system inhibitor did not affect mortality or the clinical severity of the disease.Conclusion DM is a significant risk factor for COVID-19 severity and mortality. Our findings imply that COVID-19 patients with DM, especially if elderly, require special attention and prompt intensive care.
-
Citations
Citations to this article as recorded by- Potential use of sodium glucose co-transporter 2 inhibitors during acute illness: a systematic review based on COVID-19
Carmen Tisch, Eleni Xourgia, Aristomenis Exadaktylos, Mairi Ziaka
Endocrine.2024;[Epub] CrossRef - Insulin and Metformin Administration: Unravelling the Multifaceted Association with Mortality across Various Clinical Settings Considering Type 2 Diabetes Mellitus and COVID-19
Łukasz Lewandowski, Agnieszka Bronowicka-Szydełko, Maciej Rabczyński, Dorota Bednarska-Chabowska, Joanna Adamiec-Mroczek, Adrian Doroszko, Małgorzata Trocha, Krzysztof Kujawa, Agnieszka Matera-Witkiewicz, Edwin Kuźnik, Paweł Lubieniecki, Marcin Madziarski
Biomedicines.2024; 12(3): 605. CrossRef - Pre-admission use of sodium glucose transporter-2 inhibitor (SGLT-2i) may significantly improves Covid-19 outcomes in patients with diabetes: A systematic review, meta-analysis, and meta-regression
Hikmat Permana, Theo Audi Yanto, Timotius Ivan Hariyanto
Diabetes Research and Clinical Practice.2023; 195: 110205. CrossRef - Risk phenotypes of diabetes and association with COVID-19 severity and death: an update of a living systematic review and meta-analysis
Sabrina Schlesinger, Alexander Lang, Nikoletta Christodoulou, Philipp Linnerz, Kalliopi Pafili, Oliver Kuss, Christian Herder, Manuela Neuenschwander, Janett Barbaresko, Michael Roden
Diabetologia.2023; 66(8): 1395. CrossRef - Factors influencing the severity of COVID-19 course for patients with diabetes mellitus in tashkent: a retrospective cohort study
A. V. Alieva, A. A. Djalilov, F. A. Khaydarova, A. V. Alimov, D. Z. Khalilova, V. A. Talenova, N. U. Alimova, M. D. Aripova, A. S. Sadikova
Obesity and metabolism.2023; 20(2): 92. CrossRef - Pituitary Diseases and COVID-19 Outcomes in South Korea: A Nationwide Cohort Study
Jeonghoon Ha, Kyoung Min Kim, Dong-Jun Lim, Keeho Song, Gi Hyeon Seo
Journal of Clinical Medicine.2023; 12(14): 4799. CrossRef - Epidemiological features and consequences of COVID‐19 in patients with and without gastrointestinal symptoms in southwestern Iran. A retrospective observational study
Habibollah Azarbakhsh, Leila Moftakhar, Aliasghar Valipour, Alireza Mirahmadizadeh, Hekmat Allah Moradi, Elahe Piraee
Health Science Reports.2023;[Epub] CrossRef - The Impact of Long-Term Conditions and Comorbidity Patterns on COVID-19 Infection and Hospitalisation: A Cohort Study
Yun-Ting Huang, Andrew Steptoe, Riyaz S. Patel, Esme Fuller Thomson, Dorina Cadar
Gerontology.2023; 69(10): 1200. CrossRef - Association Between Anti-diabetic Agents and Clinical Outcomes of COVID-19 in Patients with Diabetes: A Systematic Review and Meta-Analysis
Tiantian Han, Shaodi Ma, Chenyu Sun, Huimei Zhang, Guangbo Qu, Yue Chen, Ce Cheng, Eric L. Chen, Mubashir Ayaz Ahmed, Keun Young Kim, Raveena Manem, Mengshi Chen, Zhichun Guo, Hongru Yang, Yue Yan, Qin Zhou
Archives of Medical Research.2022; 53(2): 186. CrossRef - Use of DPP4i reduced odds of clinical deterioration and hyperinflammatory syndrome in COVID-19 patients with type 2 diabetes: Propensity score analysis of a territory-wide cohort in Hong Kong
Carlos K.H. Wong, David T.W. Lui, Angel Y.C. Lui, Ashley C.Y. Kwok, Marshall C.H. Low, Kristy T.K. Lau, Ivan C.H. Au, Xi Xiong, Matthew S.H. Chung, Eric H.Y. Lau, Benjamin J. Cowling
Diabetes & Metabolism.2022; 48(1): 101307. CrossRef - Dipeptidyl peptidase-4 (DPP-IV) inhibitor was associated with mortality reduction in COVID-19 — A systematic review and meta-analysis
Ahmad Fariz Malvi Zamzam Zein, Wilson Matthew Raffaello
Primary Care Diabetes.2022; 16(1): 162. CrossRef - Prevalence and impact of diabetes in hospitalized COVID‐19 patients: A systematic review and meta‐analysis
Sian A. Bradley, Maciej Banach, Negman Alvarado, Ivica Smokovski, Sonu M. M. Bhaskar
Journal of Diabetes.2022; 14(2): 144. CrossRef - Interplay between Inflammaging, Frailty and Nutrition in Covid-19: Preventive and Adjuvant Treatment Perspectives
A. Padilha de Lima, M. Macedo Rogero, T. Araujo Viel, H.M. Garay-Malpartida, I. Aprahamian, Sandra Maria Lima Ribeiro
The Journal of nutrition, health and aging.2022; 26(1): 67. CrossRef - Increase in blood glucose level and incidence of diabetic ketoacidosis in children with type 1 diabetes mellitus in the Daegu-Gyeongbuk area during the coronavirus disease 2019 (COVID-19) pandemic: a retrospective cross-sectional study
Mi Seon Lee, Rosie Lee, Cheol Woo Ko, Jung Eun Moon
Journal of Yeungnam Medical Science.2022; 39(1): 46. CrossRef - Interrelationship between 2019-nCov receptor DPP4 and diabetes mellitus targets based on protein interaction network
Qian Gao, Wenjun Zhang, Tingting Li, Guojun Yang, Wei Zhu, Naijun Chen, Huawei Jin
Scientific Reports.2022;[Epub] CrossRef - Can sodium-glucose co-transporter-2 (SGLT-2) inhibitor reduce the risk of adverse complications due to COVID-19? – Targeting hyperinflammation
Afnan Alshnbari, Iskandar Idris
Current Medical Research and Opinion.2022; 38(3): 357. CrossRef - Commentary: Mortality Risk of Antidiabetic Agents for Type 2 Diabetes With COVID-19: A Systematic Review and Meta-Analysis
Li-Min Zhao, Xie-Hui Chen, Mei Qiu
Frontiers in Endocrinology.2022;[Epub] CrossRef - COVID-19 and Diabetes
Awadhesh Kumar Singh, Kamlesh Khunti
Annual Review of Medicine.2022; 73(1): 129. CrossRef - The enzymes in COVID-19: A review
Maria Helena Menezes Estevam Alves, Layla Carvalho Mahnke, Tifany Cerqueira Macedo, Thais Ketinly dos Santos Silva, Luiz Bezerra Carvalho Junior
Biochimie.2022; 197: 38. CrossRef - IMPACT OF ANTIDIABETIC DRUGS ON RISK AND OUTCOME OF COVID-19 INFECTION: A REVIEW
Adnan A. Zainal, Marwan M. Merkhan
Military Medical Science Letters.2022; 91(2): 140. CrossRef - Does metformin affect outcomes in COVID‐19 patients with new or pre‐existing diabetes mellitus? A systematic review and meta‐analysis
Adithan Ganesh, Michael D. Randall
British Journal of Clinical Pharmacology.2022; 88(6): 2642. CrossRef - Diabetes, Metformin and the Clinical Course of Covid-19: Outcomes, Mechanisms and Suggestions on the Therapeutic Use of Metformin
Clifford J. Bailey, Mike Gwilt
Frontiers in Pharmacology.2022;[Epub] CrossRef - The Role of Diabetes and Hyperglycemia on COVID-19 Infection Course—A Narrative Review
Evangelia Tzeravini, Eleftherios Stratigakos, Chris Siafarikas, Anastasios Tentolouris, Nikolaos Tentolouris
Frontiers in Clinical Diabetes and Healthcare.2022;[Epub] CrossRef - Preadmission use of antidiabetic medications and mortality among patients with COVID-19 having type 2 diabetes: A meta-analysis
Nam Nhat Nguyen, Dung Si Ho, Hung Song Nguyen, Dang Khanh Ngan Ho, Hung-Yuan Li, Chia-Yuan Lin, Hsiao-Yean Chiu, Yang-Ching Chen
Metabolism.2022; 131: 155196. CrossRef - Glucose-Lowering Agents and COVID-19
Ah Reum Khang
The Journal of Korean Diabetes.2022; 23(1): 1. CrossRef - Impact of diabetes on COVID‐19 mortality and hospital outcomes from a global perspective: An umbrella systematic review and meta‐analysis
Stavroula Kastora, Manisha Patel, Ben Carter, Mirela Delibegovic, Phyo Kyaw Myint
Endocrinology, Diabetes & Metabolism.2022;[Epub] CrossRef - The Association Between Antidiabetic Agents and Clinical Outcomes of COVID-19 Patients With Diabetes: A Bayesian Network Meta-Analysis
Yidan Chen, Xingfei Lv, Sang Lin, Mohammad Arshad, Mengjun Dai
Frontiers in Endocrinology.2022;[Epub] CrossRef - Renin‐Angiotensin Aldosterone System Inhibitors and COVID‐19: A Systematic Review and Meta‐Analysis Revealing Critical Bias Across a Body of Observational Research
Jordan Loader, Frances C. Taylor, Erik Lampa, Johan Sundström
Journal of the American Heart Association.2022;[Epub] CrossRef - Diabetes and SARS-CoV-2–Is There a Mutual Connection?
Anna P. Jedrzejak, Edyta K. Urbaniak, Jadwiga A. Wasko, Natalia Ziojla, Malgorzata Borowiak
Frontiers in Cell and Developmental Biology.2022;[Epub] CrossRef - The relationship of age, sex and prothrombin time related to the severity and mortality of COVID-19 patients with diabetes mellitus: a systematic review and meta analysis
Audrey Fabianisa Mirza, Ceria Halim, Mutiara Indah Sari
F1000Research.2022; 11: 729. CrossRef - Are lipid ratios and triglyceride-glucose index associated with critical care outcomes in COVID-19 patients?
Marzieh Rohani-Rasaf, Kosar Mirjalili, Akram Vatannejad, Maryam Teimouri, Xiao-Feng Yang
PLOS ONE.2022; 17(8): e0272000. CrossRef - Early glycaemic variability increases 28-day mortality and prolongs intensive care unit stay in critically ill patients with pneumonia
Seong Ho Kim, Ji Young Kim, Eun Song Kim, Il Rae Park, Eun Yeong Ha, Seung Min Chung, Jun Sung Moon, Ji Sung Yoon, Kyu Chang Won, Hyoung Woo Lee
Annals of Medicine.2022; 54(1): 2724. CrossRef - Dipeptidyl peptidase 4 inhibitors in COVID-19: Beyond glycemic control
Niya Narayanan, Dukhabandhu Naik, Jayaprakash Sahoo, Sadishkumar Kamalanathan
World Journal of Virology.2022; 11(6): 399. CrossRef - Prevalencia de secuelas en pacientes con diabetes mellitus tipo 2 sobrevivientes al COVID-19
Gianela M. Cancino-Castillo, Miguel A. Tresierra-Ayala, Jorge L. Campos-Reyna, Jaime Rosales-Rimache
REVISTA MÉDICA VALLEJIANA/ Vallejian Medical Journal.2022; 11(2): 48. CrossRef - Predictors of adverse in-hospital outcome and recovery in patients with diabetes mellitus and COVID-19 pneumonia in Iraq
Hussein Nafakhi, Mohammed Alareedh, Karrar Al-Buthabhak, Foaad Shaghee, Ahmed Nafakhi, Samet Kasim
Diabetes & Metabolic Syndrome: Clinical Research & Reviews.2021; 15(1): 33. CrossRef - Non-insulin anti-diabetic agents in patients with type 2 diabetes and COVID-19: A Critical Appraisal of Literature
Awadhesh Kumar Singh, Ritu Singh, Banshi Saboo, Anoop Misra
Diabetes & Metabolic Syndrome: Clinical Research & Reviews.2021; 15(1): 159. CrossRef - COVID-19 associated with diabetes and other noncommunicable diseases led to a global health crisis
Mark Thomaz Ugliara Barone, Belinda Ngongo, Simone Bega Harnik, Lucas Xavier de Oliveira, Dániel Végh, Patrícia Vieira de Luca, Hermelinda Cordeiro Pedrosa, Franco Giraudo, Roque Cardona-Hernandez, Nayanjeet Chaudhury, Luiz Menna-Barreto
Diabetes Research and Clinical Practice.2021; 171: 108587. CrossRef - A meta-analysis on the preadmission use of DPP-4 inhibitors and risk of a fatal or severe course of illness in patients with COVID-19
Chia Siang Kow, Syed Shahzad Hasan
Therapies.2021; 76(4): 361. CrossRef - Disentangling conflicting evidence on DPP-4 inhibitors and outcomes of COVID-19: narrative review and meta-analysis
B. M. Bonora, A. Avogaro, G. P. Fadini
Journal of Endocrinological Investigation.2021; 44(7): 1379. CrossRef - Prognostic bioindicators in severe COVID-19 patients
L. Bergantini, E. Bargagli, M. d'Alessandro, R.M. Refini, P. Cameli, L. Galasso, C. Scapellato, F. Montagnani, S. Scolletta, F. Franchi, S. Valente, D. Bennett, G. Sebastiani, B. Frediani, F. Dotta
Cytokine.2021; 141: 155455. CrossRef - Epidemiological characteristics and outcomes of COVID-19 in diabetic versus non-diabetic patients
Leila Moftakhar, Parisa Moftakhar, Elahe Piraee, Haleh Ghaem, Aliasghar Valipour, Habibollah Azarbakhsh
International Journal of Diabetes in Developing Countries.2021; 41(3): 383. CrossRef - DPP-4 inhibition and COVID-19: From initial concerns to recent expectations
André J. Scheen
Diabetes & Metabolism.2021; 47(2): 101213. CrossRef - Use of dipeptidyl peptidase‐4 inhibitors and prognosis of COVID‐19 in hospitalized patients with type 2 diabetes: A propensity score analysis from the CORONADO study
Ronan Roussel, Patrice Darmon, Matthieu Pichelin, Thomas Goronflot, Yawa Abouleka, Leila Ait Bachir, Ingrid Allix, Deborah Ancelle, Sara Barraud, Lyse Bordier, Aurélie Carlier, Nicolas Chevalier, Christine Coffin‐Boutreux, Emmanuel Cosson, Anne Dorange, O
Diabetes, Obesity and Metabolism.2021; 23(5): 1162. CrossRef - Dipeptidyl peptidase-4 inhibitor use and mortality in COVID-19 patients with diabetes mellitus: an updated systematic review and meta-analysis
Rimesh Pal, Mainak Banerjee, Soham Mukherjee, Ranjitpal Singh Bhogal, Amanpreet Kaur, Sanjay K. Bhadada
Therapeutic Advances in Endocrinology and Metabolism.2021; 12: 204201882199648. CrossRef - Renin–angiotensin-system inhibitors and all-cause mortality in patients with COVID-19: a systematic review and meta-analysis of observational studies
Chirag Bavishi, Paul K. Whelton, Giuseppe Mancia, Giovanni Corrao, Franz H. Messerli
Journal of Hypertension.2021; 39(4): 784. CrossRef - Evaluation of the Current Therapeutic Approaches for COVID-19: A Systematic Review and a Meta-analysis
Zeinab Abdelrahman, Qian Liu, Shanmei Jiang, Mengyuan Li, Qingrong Sun, Yue Zhang, Xiaosheng Wang
Frontiers in Pharmacology.2021;[Epub] CrossRef - Dipeptidyl peptidase 4 (DPP4) inhibitor and outcome from coronavirus disease 2019 (COVID-19) in diabetic patients: a systematic review, meta-analysis, and meta-regression
Timotius Ivan Hariyanto, Andree Kurniawan
Journal of Diabetes & Metabolic Disorders.2021; 20(1): 543. CrossRef - Impact of diabetes mellitus on in-hospital mortality in adult patients with COVID-19: a systematic review and meta-analysis
Halla Kaminska, Lukasz Szarpak, Dariusz Kosior, Wojciech Wieczorek, Agnieszka Szarpak, Mahdi Al-Jeabory, Wladyslaw Gawel, Aleksandra Gasecka, Milosz J. Jaguszewski, Przemyslawa Jarosz-Chobot
Acta Diabetologica.2021; 58(8): 1101. CrossRef - Dipeptidyl peptidase-4 (DPP-4) inhibitor and mortality in coronavirus disease 2019 (COVID-19) – A systematic review, meta-analysis, and meta-regression
Iis Inayati Rakhmat, Yudith Yunia Kusmala, Dewi Ratih Handayani, Henny Juliastuti, Eka Noneng Nawangsih, Arief Wibowo, Michael Anthonius Lim, Raymond Pranata
Diabetes & Metabolic Syndrome: Clinical Research & Reviews.2021; 15(3): 777. CrossRef - Post-infection depressive, anxiety and post-traumatic stress symptoms: A prospective cohort study in patients with mild COVID-19
Flavia Ismael, João C.S. Bizario, Tatiane Battagin, Beatriz Zaramella, Fabio E. Leal, Julio Torales, Antonio Ventriglio, Megan E. Marziali, Silvia S. Martins, João M. Castaldelli-Maia
Progress in Neuro-Psychopharmacology and Biological Psychiatry.2021; 111: 110341. CrossRef - Managing diabetes in diabetic patients with COVID: where do we start from?
Angelo Avogaro, Benedetta Bonora, Gian Paolo Fadini
Acta Diabetologica.2021; 58(11): 1441. CrossRef - Is diabetes mellitus a wrongdoer to COVID-19 severity?
Sanjib Sarkar, Dibyendu Das, Sawlang Borsingh Wann, Jatin Kalita, Prasenjit Manna
Diabetes Research and Clinical Practice.2021; 178: 108936. CrossRef - Dipeptidyl Peptidase 4 Inhibitor, an Update
Ju Hee Lee
The Journal of Korean Diabetes.2021; 22(2): 91. CrossRef - Correlation Analysis Between Serum Uric Acid, Prealbumin Level, Lactate Dehydrogenase, and Severity of COVID-19
Zhenmu Jin, Mo Zheng, Jichan Shi, Xinchun Ye, Fang Cheng, Que-Lu Chen, Jianping Huang, Xian-Gao Jiang
Frontiers in Molecular Biosciences.2021;[Epub] CrossRef - Association Between Glucagon-Like Peptide 1 Receptor Agonist and Sodium–Glucose Cotransporter 2 Inhibitor Use and COVID-19 Outcomes
Anna R. Kahkoska, Trine Julie Abrahamsen, G. Caleb Alexander, Tellen D. Bennett, Christopher G. Chute, Melissa A. Haendel, Klara R. Klein, Hemalkumar Mehta, Joshua D. Miller, Richard A. Moffitt, Til Stürmer, Kajsa Kvist, John B. Buse, Tim Q. Duong
Diabetes Care.2021; 44(7): 1564. CrossRef - The effect of metformin on mortality and severity in COVID-19 patients with diabetes mellitus
Wenxing Yang, Xuehong Sun, Jun Zhang, Kui Zhang
Diabetes Research and Clinical Practice.2021; 178: 108977. CrossRef - Renin‐Angiotensin Aldosterone System Inhibitors in Primary Prevention and COVID‐19
Jordan Loader, Erik Lampa, Stefan Gustafsson, Thomas Cars, Johan Sundström
Journal of the American Heart Association.2021;[Epub] CrossRef - Factors influencing on development of COVID-19 pneumonia and association with oral anti-diabetic drugs in hospitalized patients with diabetes mellitus
Ayça Elibol, Didem Eren, Macide Deniz Erdoğan, Merve Elmaağaç, Oguzhan Sıtkı Dizdar, İlhami Çelik, Ali İhsan Günal
Primary Care Diabetes.2021; 15(5): 806. CrossRef - Aging & COVID-19 susceptibility, disease severity, and clinical outcomes: The role of entangled risk factors
Melina Farshbafnadi, Sara Kamali Zonouzi, Mohammadmahdi Sabahi, Mahsa Dolatshahi, Mohammad Hadi Aarabi
Experimental Gerontology.2021; 154: 111507. CrossRef - Classical and Counter-Regulatory Renin–Angiotensin System: Potential Key Roles in COVID-19 Pathophysiology
Moudhi Almutlaq, Abir Abdullah Alamro, Fayhan Alroqi, Tlili Barhoumi
CJC Open.2021; 3(8): 1060. CrossRef - Metformin in Patients With COVID-19: A Systematic Review and Meta-Analysis
Yin Li, Xue Yang, Peijing Yan, Tong Sun, Zhi Zeng, Sheyu Li
Frontiers in Medicine.2021;[Epub] CrossRef - Pre-existing health conditions and severe COVID-19 outcomes: an umbrella review approach and meta-analysis of global evidence
Marina Treskova-Schwarzbach, Laura Haas, Sarah Reda, Antonia Pilic, Anna Borodova, Kasra Karimi, Judith Koch, Teresa Nygren, Stefan Scholz, Viktoria Schönfeld, Sabine Vygen-Bonnet, Ole Wichmann, Thomas Harder
BMC Medicine.2021;[Epub] CrossRef - COVID-19 Vaccination for Endocrine Patients: A Position Statement from the Korean Endocrine Society
Cheol Ryong Ku, Kyong Yeun Jung, Chang Ho Ahn, Jun Sung Moon, Ju Hee Lee, Eun Heui Kim, Hyemi Kwon, Hee Kyung Kim, Sunghwan Suh, Sangmo Hong, Jeonghoon Ha, Eun Roh, Jin Hwa Kim, Mi-kyung Kim
Endocrinology and Metabolism.2021; 36(4): 757. CrossRef - High Fibrosis-4 Index Is Related with Worse Clinical Outcome in Patients with Coronavirus Disease 2019 and Diabetes Mellitus: A Multicenter Observational Study
Sung-Woo Kim, Jae-Han Jeon, Jun Sung Moon, Mi Kyung Kim
Endocrinology and Metabolism.2021; 36(4): 800. CrossRef - Mortality Risk of Antidiabetic Agents for Type 2 Diabetes With COVID-19: A Systematic Review and Meta-Analysis
Chengxia Kan, Yang Zhang, Fang Han, Qian Xu, Tongtong Ye, Ningning Hou, Xiaodong Sun
Frontiers in Endocrinology.2021;[Epub] CrossRef - Analysis of influence of background therapy for comorbidities in the period before infection on the risk of the lethal COVID outcome. Data from the international ACTIV SARS-CoV-2 registry («Analysis of chronic non-infectious diseases dynamics after COVID-
E. I. Tarlovskaya, A. G. Arutyunov, A. O. Konradi, Yu. M. Lopatin, A. P. Rebrov, S. N. Tereshchenko, A. I. Chesnikova, H. G. Hayrapetyan, A. P. Babin, I. G. Bakulin, N. V. Bakulina, L. A. Balykova, A. S. Blagonravova, M. V. Boldina, A. R. Vaisberg, A. S.
Kardiologiia.2021; 61(9): 20. CrossRef - Association of clinical characteristics, antidiabetic and cardiovascular agents with diabetes mellitus and COVID-19: a 7-month follow-up cohort study
Marzieh Pazoki, Fatemeh Chichagi, Azar Hadadi, Samira Kafan, Mahnaz Montazeri, Sina Kazemian, Arya Aminorroaya, Mehdi Ebrahimi, Haleh Ashraf, Mojgan Mirabdolhagh Hazaveh, Mohammad Reza Khajavi, Reza Shariat Moharari, Seyed Hamidreza Sharifnia, Shahrokh Ka
Journal of Diabetes & Metabolic Disorders.2021; 20(2): 1545. CrossRef - COVID-19 and Diabetes: A Comprehensive Review of Angiotensin Converting Enzyme 2, Mutual Effects and Pharmacotherapy
Lingli Xie, Ziying Zhang, Qian Wang, Yangwen Chen, Dexue Lu, Weihua Wu
Frontiers in Endocrinology.2021;[Epub] CrossRef - Impact of Diabetes on COVID-19 Mortality and Hospital Outcomes, a Global Perspective: An ONTOP Systematic Review and Meta-Analysis
Stavroula Kastora, Manisha Patel, Ben Carter, Mirela Delibegovic, Phyo Kyaw Myint
SSRN Electronic Journal .2021;[Epub] CrossRef - Decision Trees: Predictions of Global Vulnerability to Coronavirus Outbreaks
Moacir José da Silva
SSRN Electronic Journal .2020;[Epub] CrossRef - The potential association between common comorbidities and severity and mortality of coronavirus disease 2019: A pooled analysis
Liman Luo, Menglu Fu, Yuanyuan Li, Shuiqing Hu, Jinlan Luo, Zhihui Chen, Jing Yu, Wenhua Li, Ruolan Dong, Yan Yang, Ling Tu, Xizhen Xu
Clinical Cardiology.2020; 43(12): 1478. CrossRef - The Effect of Metformin Consumption on Mortality in Hospitalized COVID-19 patients: a systematic review and meta-analysis
Antonia Anna Lukito, Raymond Pranata, Joshua Henrina, Michael Anthonius Lim, Sherly Lawrensia, Ketut Suastika
Diabetes & Metabolic Syndrome: Clinical Research & Reviews.2020; 14(6): 2177. CrossRef - Risk Factors on the Progression to Clinical Outcomes of COVID-19 Patients in South Korea: Using National Data
Seon-Rye Kim, Seoul-Hee Nam, Yu-Rin Kim
International Journal of Environmental Research and Public Health.2020; 17(23): 8847. CrossRef - Clinical Outcomes of COVID-19 Patients with Type 2 Diabetes: A Population-Based Study in Korea
Ji Hong You, Sang Ah Lee, Sung-Youn Chun, Sun Ok Song, Byung-Wan Lee, Dae Jung Kim, Edward J. Boyko
Endocrinology and Metabolism.2020; 35(4): 901. CrossRef
- Potential use of sodium glucose co-transporter 2 inhibitors during acute illness: a systematic review based on COVID-19
- Guideline/Fact Sheet
- Non-Alcoholic Fatty Liver Disease in Patients with Type 2 Diabetes Mellitus: A Position Statement of the Fatty Liver Research Group of the Korean Diabetes Association
- Byung-Wan Lee, Yong-ho Lee, Cheol-Young Park, Eun-Jung Rhee, Won-Young Lee, Nan-Hee Kim, Kyung Mook Choi, Keun-Gyu Park, Yeon-Kyung Choi, Bong-Soo Cha, Dae Ho Lee, Korean Diabetes Association (KDA) Fatty Liver Research Group
- Diabetes Metab J. 2020;44(3):382-401. Published online May 11, 2020
- DOI: https://doi.org/10.4093/dmj.2020.0010
- 12,352 View
- 338 Download
- 42 Web of Science
- 42 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader This clinical practice position statement, a product of the Fatty Liver Research Group of the Korean Diabetes Association, proposes recommendations for the diagnosis, progression and/or severity assessment, management, and follow-up of non-alcoholic fatty liver disease (NAFLD) in patients with type 2 diabetes mellitus (T2DM). Patients with both T2DM and NAFLD have an increased risk of non-alcoholic steatohepatitis (NASH) and fibrosis and a higher risk of cardiovascular diseases and diabetic complications compared to those without NAFLD. With regards to the evaluation of patients with T2DM and NAFLD, ultrasonography-based stepwise approaches using noninvasive biomarker models such as fibrosis-4 or the NAFLD fibrosis score as well as imaging studies such as vibration-controlled transient elastography with controlled attenuation parameter or magnetic resonance imaging-proton density fat fraction are recommended. After the diagnosis of NAFLD, the stage of fibrosis needs to be assessed appropriately. For management, weight reduction achieved by lifestyle modification has proven beneficial and is recommended in combination with antidiabetic agent(s). Evidence that some antidiabetic agents improve NAFLD/NASH with fibrosis in patients with T2DM is emerging. However, there are currently no definite pharmacologic treatments for NAFLD in patients with T2DM. For specific cases, bariatric surgery may be an option if indicated.
-
Citations
Citations to this article as recorded by- A combined extract containing Schisandra chinensis (SCE) reduced hepatic triglyceride accumulation in rats fed a high-sucrose diet
Haneul Lee, Eun Young Kang, Joowon Lee, Yejin Kim, Sumin Kang, Hayoon Kim, Hyun Kyung Kim, Gyoungok Gang, Sang-gil Lee, Cao Lei, Gwang-woong Go
Food Science and Biotechnology.2024; 33(6): 1449. CrossRef - Association of non-alcoholic fatty liver disease with cardiovascular disease and all cause death in patients with type 2 diabetes mellitus: nationwide population based study
Kyung-Soo Kim, Sangmo Hong, Kyungdo Han, Cheol-Young Park
BMJ.2024; : e076388. CrossRef - Risk Scores for Prediction of Major Cardiovascular Events in Non-Alcoholic Fatty Liver Disease: A No Man’s Land?
Liliana Gheorghe, Roxana Nemteanu, Andreea Clim, Gina Eosefina Botnariu, Irina Iuliana Costache, Alina Plesa
Life.2023; 13(4): 857. CrossRef - Increased expression of sodium-glucose cotransporter 2 and O-GlcNAcylation in hepatocytes drives non-alcoholic steatohepatitis
Hye Jin Chun, Eun Ran Kim, Minyoung Lee, Da Hyun Choi, Soo Hyun Kim, Eugene Shin, Jin-Hong Kim, Jin Won Cho, Dai Hoon Han, Bong-Soo Cha, Yong-ho Lee
Metabolism.2023; 145: 155612. CrossRef - Association between fatty liver index and risk of end-stage renal disease stratified by kidney function in patients with type 2 diabetes: A nationwide population-based study
Goh Eun Chung, Kyungdo Han, Kyu-Na Lee, Jung Ho Bae, Sun Young Yang, Su-Yeon Choi, Jeong Yoon Yim, Nam Ju Heo
Diabetes & Metabolism.2023; 49(4): 101454. CrossRef - Comparison of glucagon-like peptide-1 receptor agonists and thiazolidinediones on treating nonalcoholic fatty liver disease: A network meta-analysis
Min Jeong Park, Hayeon Kim, Myeong Gyu Kim, Kyungim Kim
Clinical and Molecular Hepatology.2023; 29(3): 693. CrossRef - Histological analysis of hypoglycemic agents on liver fibrosis in patients with non-alcoholic fatty liver disease: a systematic review
Qingxing Xie, Xiaohui Pan, Xinyue Zhang, Jinfang Ma, Ge Peng, Nanwei Tong
Chinese Medical Journal.2023; 136(16): 2014. CrossRef - Hepatotropc effects of glucose-lowering drugs: non-alcoholic fatty liver disease in focus
E. V. Uzhakova, Z. E. Zshanko, E. N. Smirnova
Experimental and Clinical Gastroenterology.2023; (6): 121. CrossRef - Complementary effects of dapagliflozin and lobeglitazone on metabolism in a diet-induced obese mouse model
Yun Kyung Lee, Tae Jung Oh, Ji In Lee, Bo Yoon Choi, Hyen Chung Cho, Hak Chul Jang, Sung Hee Choi
European Journal of Pharmacology.2023; 957: 175946. CrossRef - Hepatic T-cell senescence and exhaustion are implicated in the progression of fatty liver disease in patients with type 2 diabetes and mouse model with nonalcoholic steatohepatitis
Byeong Chang Sim, Yea Eun Kang, Sun Kyoung You, Seong Eun Lee, Ha Thi Nga, Ho Yeop Lee, Thi Linh Nguyen, Ji Sun Moon, Jingwen Tian, Hyo Ju Jang, Jeong Eun Lee, Hyon-Seung Yi
Cell Death & Disease.2023;[Epub] CrossRef - 2023 Clinical Practice Guidelines for Diabetes Mellitus of the Korean Diabetes Association
Jong Han Choi, Kyung Ae Lee, Joon Ho Moon, Suk Chon, Dae Jung Kim, Hyun Jin Kim, Nan Hee Kim, Ji A Seo, Mee Kyoung Kim, Jeong Hyun Lim, YoonJu Song, Ye Seul Yang, Jae Hyeon Kim, You-Bin Lee, Junghyun Noh, Kyu Yeon Hur, Jong Suk Park, Sang Youl Rhee, Hae J
Diabetes & Metabolism Journal.2023; 47(5): 575. CrossRef - Circ_0004535/miR-1827/CASP8 network involved in type 2 diabetes mellitus with nonalcoholic fatty liver disease
Min Li, Ai Zeng, Xinle Tang, Hui Xu, Wei Xiong, Yanying Guo
Scientific Reports.2023;[Epub] CrossRef - Stratification by obesity class, rather than age, can identify a higher percent of children at risk for non‐alcoholic fatty liver disease and metabolic dysfunction
Aurelia Radulescu, Adam J. Dugan, Mary Killian, Suzanna L. Attia, Marialena Mouzaki, George J. Fuchs, Rohit Kohli, Henrietta Bada, Philip A. Kern, Samir Softic
Pediatric Obesity.2022;[Epub] CrossRef - Renal Tubular Damage Marker, Urinary N-acetyl-β-D-Glucosaminidase, as a Predictive Marker of Hepatic Fibrosis in Type 2 Diabetes Mellitus
Hae Kyung Kim, Minyoung Lee, Yong-ho Lee, Eun Seok Kang, Bong-Soo Cha, Byung-Wan Lee
Diabetes & Metabolism Journal.2022; 46(1): 104. CrossRef - Dulaglutide Ameliorates Palmitic Acid-Induced Hepatic Steatosis by Activating FAM3A Signaling Pathway
Jinmi Lee, Seok-Woo Hong, Min-Jeong Kim, Sun Joon Moon, Hyemi Kwon, Se Eun Park, Eun-Jung Rhee, Won-Young Lee
Endocrinology and Metabolism.2022; 37(1): 74. CrossRef - State-of-the-Art Overview of the Pharmacological Treatment of Non-Alcoholic Steatohepatitis
Yongin Cho, Yong-ho Lee
Endocrinology and Metabolism.2022; 37(1): 38. CrossRef - Ezetimibe combination therapy with statin for non-alcoholic fatty liver disease: an open-label randomized controlled trial (ESSENTIAL study)
Yongin Cho, Hyungjin Rhee, Young-eun Kim, Minyoung Lee, Byung-Wan Lee, Eun Seok Kang, Bong-Soo Cha, Jin-Young Choi, Yong-ho Lee
BMC Medicine.2022;[Epub] CrossRef - Obesity is an important determinant of severity in newly defined metabolic dysfunction-associated fatty liver disease
Ji Hye Huh, Kwang Joon Kim, Seung Up Kim, Bong-Soo Cha, Byung-Wan Lee
Hepatobiliary & Pancreatic Diseases International.2022; 21(3): 241. CrossRef - Anti-Obesity and Anti-Hyperglycemic Effects of Meretrix lusoria Protamex Hydrolysate in ob/ob Mice
Min Ju Kim, Ramakrishna Chilakala, Hee Geun Jo, Seung-Jae Lee, Dong-Sung Lee, Sun Hee Cheong
International Journal of Molecular Sciences.2022; 23(7): 4015. CrossRef - The associations of hepatic steatosis and fibrosis using fatty liver index and BARD score with cardiovascular outcomes and mortality in patients with new-onset type 2 diabetes: a nationwide cohort study
Jiyun Park, Gyuri Kim, Bong-Sung Kim, Kyung-Do Han, So Yoon Kwon, So Hee Park, You-Bin Lee, Sang-Man Jin, Jae Hyeon Kim
Cardiovascular Diabetology.2022;[Epub] CrossRef - Cumulative Exposure to High γ-Glutamyl Transferase Level and Risk of Diabetes: A Nationwide Population-Based Study
Ji-Yeon Park, Kyungdo Han, Hun-Sung Kim, Jae-Hyoung Cho, Kun-Ho Yoon, Mee Kyoung Kim, Seung-Hwan Lee
Endocrinology and Metabolism.2022; 37(2): 272. CrossRef - SGLT-2 inhibitors and GLP-1 receptor agonists in metabolic dysfunction-associated fatty liver disease
Jun Sung Moon, Jun Hwa Hong, Yong Jin Jung, Ele Ferrannini, Michael A. Nauck, Soo Lim
Trends in Endocrinology & Metabolism.2022; 33(6): 424. CrossRef - Plasma Aldo-Keto Reductase Family 1 Member B10 as a Biomarker Performs Well in the Diagnosis of Nonalcoholic Steatohepatitis and Fibrosis
Aron Park, Seung Joon Choi, Sungjin Park, Seong Min Kim, Hye Eun Lee, Minjae Joo, Kyoung Kon Kim, Doojin Kim, Dong Hae Chung, Jae Been Im, Jaehun Jung, Seung Kak Shin, Byung-Chul Oh, Cheolsoo Choi, Seungyoon Nam, Dae Ho Lee
International Journal of Molecular Sciences.2022; 23(9): 5035. CrossRef - Chinese Herbal Medicine for Type 2 Diabetes Mellitus With Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis
Sihan Peng, Lu Liu, Ziyan Xie, Xiyu Zhang, Chunguang Xie, Sha Ye, Xiangeng Zhang, Xiaoli Liang, Hongyan Wang, Ya Liu
Frontiers in Pharmacology.2022;[Epub] CrossRef - Non-alcoholic fatty liver disease and sarcopenia is associated with the risk of albuminuria independent of insulin resistance, and obesity
Eugene Han, Mi Kyung Kim, Seung-Soon Im, Byoung Kuk Jang, Hye Soon Kim
Journal of Diabetes and its Complications.2022; 36(8): 108253. CrossRef - Extra-Glycemic Effects of Anti-Diabetic Medications: Two Birds with One Stone?
Eun-Jung Rhee
Endocrinology and Metabolism.2022; 37(3): 415. CrossRef - Plasma Metabolomics and Machine Learning-Driven Novel Diagnostic Signature for Non-Alcoholic Steatohepatitis
Moongi Ji, Yunju Jo, Seung Joon Choi, Seong Min Kim, Kyoung Kon Kim, Byung-Chul Oh, Dongryeol Ryu, Man-Jeong Paik, Dae Ho Lee
Biomedicines.2022; 10(7): 1669. CrossRef - Association Between DPP4 Inhibitor Use and the Incidence of Cirrhosis, ESRD, and Some Cancers in Patients With Diabetes
Yewon Na, Soo Wan Kim, Ie Byung Park, Soo Jung Choi, Seungyoon Nam, Jaehun Jung, Dae Ho Lee
The Journal of Clinical Endocrinology & Metabolism.2022; 107(11): 3022. CrossRef - Nonalcoholic fatty liver disease is associated with early left ventricular diastolic dysfunction in patients with type 2 diabeteS
Walaa Sheba, Eman Morsy, Salah Altahan, Mona Ayaad, Sameh A. Lashen
Alexandria Journal of Medicine.2022; 58(1): 117. CrossRef - The association between changes in hepatic steatosis and hepatic fibrosis with cardiovascular outcomes and mortality in patients with New-Onset type 2 Diabetes: A nationwide cohort study
Jiyun Park, Gyuri Kim, Bong-Sung Kim, Kyung-Do Han, So Yoon Kwon, So Hee Park, You-Bin Lee, Sang-Man Jin, Jae Hyeon Kim
Diabetes Research and Clinical Practice.2022; 194: 110191. CrossRef - Advanced Liver Fibrosis Is Associated with Chronic Kidney Disease in Patients with Type 2 Diabetes Mellitus and Nonalcoholic Fatty Liver Disease (Diabetes Metab J 2022;46:630-9)
Ji Hye Huh
Diabetes & Metabolism Journal.2022; 46(6): 953. CrossRef - The association of fatty liver index and BARD score with all-cause and cause-specific mortality in patients with type 2 diabetes mellitus: a nationwide population-based study
Goh Eun Chung, Su-Min Jeong, Eun Ju Cho, Ji Won Yoon, Jeong-Ju Yoo, Yuri Cho, Kyu-na Lee, Dong Wook Shin, Yoon Jun Kim, Jung-Hwan Yoon, Kyungdo Han, Su Jong Yu
Cardiovascular Diabetology.2022;[Epub] CrossRef - Non-Albumin Proteinuria (NAP) as a Complementary Marker for Diabetic Kidney Disease (DKD)
Jaehyun Bae, Young Jun Won, Byung-Wan Lee
Life.2021; 11(3): 224. CrossRef - Diabetes Mellitus and Non-Alcoholic Fatty Liver Disease: Diagnosis and Treatment
Sook Jung Lee, Byung-Wan Lee
The Journal of Korean Diabetes.2021; 22(1): 38. CrossRef - Non-alcoholic fatty liver disease
Elizabeth E Powell, Vincent Wai-Sun Wong, Mary Rinella
The Lancet.2021; 397(10290): 2212. CrossRef - Allopurinol ameliorates high fructose diet induced hepatic steatosis in diabetic rats through modulation of lipid metabolism, inflammation, and ER stress pathway
In-Jin Cho, Da-Hee Oh, Jin Yoo, You-Cheol Hwang, Kyu Jeung Ahn, Ho-Yeon Chung, Soung Won Jeong, Ju-Young Moon, Sang-Ho Lee, Sung-Jig Lim, In-Kyung Jeong
Scientific Reports.2021;[Epub] CrossRef - Patient Management in Non-Alcoholic Fatty Liver Disease and Type 2 Diabetes Mellitus
A. E. Bagriy, A. D. Zubov, M. V. Khomenko, E. S. Mikhailichenko, E. A. Pylaeva, N. A. Khaustova, E. V. Bryukhovetskaya
Russian Journal of Gastroenterology, Hepatology, Coloproctology.2021; 31(2): 14. CrossRef - Liver Fibrosis Indices for the Prediction of Mortality in Korean Subjects: A 16-Year Prospective Cohort Study
Tae Jung Oh, Kyuho Kim, Jae Hoon Moon, Sung Hee Choi, Nam H Cho, Hak Chul Jang
Journal of the Endocrine Society.2021;[Epub] CrossRef - Reduced Rank Regression-Derived Dietary Patterns Related to the Fatty Liver Index and Associations with Type 2 Diabetes Mellitus among Ghanaian Populations under Transition: The RODAM Study
Tracy Bonsu Osei, Anne-Marieke van Dijk, Sjoerd Dingerink, Felix Patience Chilunga, Erik Beune, Karlijn Anna Catharina Meeks, Silver Bahendeka, Matthias Bernd Schulze, Charles Agyemang, Mary Nicolaou, Adriaan Georgius Holleboom, Ina Danquah
Nutrients.2021; 13(11): 3679. CrossRef - Efficacy and Safety of GLP-1 Receptor Agonists in Patients With Type 2 Diabetes Mellitus and Non-Alcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis
Yuan Zhu, Jiao Xu, Dong Zhang, Xingyu Mu, Yi Shi, Shangtao Chen, Zengxiang Wu, Shuangqing Li
Frontiers in Endocrinology.2021;[Epub] CrossRef - Liver fibrosis indices are related to diabetic peripheral neuropathy in individuals with type 2 diabetes
Kyuho Kim, Tae Jung Oh, Hyen Chung Cho, Yun Kyung Lee, Chang Ho Ahn, Bo Kyung Koo, Jae Hoon Moon, Sung Hee Choi, Hak Chul Jang
Scientific Reports.2021;[Epub] CrossRef - Evaluation of Grapefruit Juice in terms of Interleukin 18 Gene Expression in Rats with Fatty Liver and Healthy Rats
Simin Bahmanpoor, Noosha Zia-Jahromi
journal of ilam university of medical sciences.2021; 29(4): 74. CrossRef
- A combined extract containing Schisandra chinensis (SCE) reduced hepatic triglyceride accumulation in rats fed a high-sucrose diet
- Drug/Regimen
- Evogliptin, a Dipeptidyl Peptidase-4 Inhibitor, Attenuates Renal Fibrosis Caused by Unilateral Ureteral Obstruction in Mice
- Mi-Jin Kim, Na-young Kim, Yun-A Jung, Seunghyeong Lee, Gwon-Soo Jung, Jung-Guk Kim, In-Kyu Lee, Sungwoo Lee, Yeon-Kyung Choi, Keun-Gyu Park
- Diabetes Metab J. 2020;44(1):186-192. Published online October 31, 2019
- DOI: https://doi.org/10.4093/dmj.2018.0271
- 5,667 View
- 97 Download
- 10 Web of Science
- 10 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader Renal fibrosis is considered to be the final common outcome of chronic kidney disease. Dipeptidyl peptidase-4 (DPP-4) inhibitors have demonstrated protective effects against diabetic kidney disease. However, the anti-fibrotic effect of evogliptin, a DPP-4 inhibitor, has not been studied. Here, we report the beneficial effects of evogliptin on unilateral ureteral obstruction (UUO)-induced renal fibrosis in mice. Evogliptin attenuated UUO-induced renal atrophy and tubulointerstitial fibrosis. Immunohistochemistry and Western blotting demonstrated that evogliptin treatment inhibits pro-fibrotic gene expressions and extracellular matrix production.
In vitro findings showed that the beneficial effects of evogliptin on renal fibrosis are mediated by inhibition of the transforming growth factor-β/Smad3 signaling pathway. The present study demonstrates that evogliptin is protective against UUO-induced renal fibrosis, suggesting that its clinical applications could extend to the treatment of kidney disease of non-diabetic origin.-
Citations
Citations to this article as recorded by- Targeting cluster of differentiation 26 / dipeptidyl peptidase 4 (CD26/DPP4) in organ fibrosis
Birte Ohm, Isabelle Moneke, Wolfgang Jungraithmayr
British Journal of Pharmacology.2023; 180(22): 2846. CrossRef - Linagliptin ameliorates pulmonary fibrosis in systemic sclerosis mouse model via inhibition of endothelial-to-mesenchymal transition
Biwei Pei, Na Zhang, Tingting Pang, Gengyun Sun
Molecular and Cellular Biochemistry.2022; 477(4): 995. CrossRef - Association Between DPP4 Inhibitor Use and the Incidence of Cirrhosis, ESRD, and Some Cancers in Patients With Diabetes
Yewon Na, Soo Wan Kim, Ie Byung Park, Soo Jung Choi, Seungyoon Nam, Jaehun Jung, Dae Ho Lee
The Journal of Clinical Endocrinology & Metabolism.2022; 107(11): 3022. CrossRef - Evogliptin Directly Inhibits Inflammatory and Fibrotic Signaling in Isolated Liver Cells
Hye-Young Seo, So-Hee Lee, Eugene Han, Jae Seok Hwang, Sol Han, Mi Kyung Kim, Byoung Kuk Jang
International Journal of Molecular Sciences.2022; 23(19): 11636. CrossRef - Optimization and validation of a fluorogenic dipeptidyl peptidase 4 enzymatic assay in human plasma
Hyunyee Yoon, Su Hee Cho, Yu Rim Seo, Kyung-Sang Yu, Sung Sup Park, Moon Jung Song
Analytical Biochemistry.2021; 612: 113952. CrossRef - Use of Anti-Diabetic Agents in Non-Diabetic Kidney Disease: From Bench to Bedside
Sungjin Chung, Gheun-Ho Kim
Life.2021; 11(5): 389. CrossRef - Targeting Dermal Fibroblast Subtypes in Antifibrotic Therapy: Surface Marker as a Cellular Identity or a Functional Entity?
Xin Huang, Yimin Khoong, Chengyao Han, Dai Su, Hao Ma, Shuchen Gu, Qingfeng Li, Tao Zan
Frontiers in Physiology.2021;[Epub] CrossRef - Efficacy and safety of evogliptin treatment in patients with type 2 diabetes: A multicentre, active‐controlled, randomized, double‐blind study with open‐label extension (the EVERGREEN study)
Gyuri Kim, Soo Lim, Hyuk‐Sang Kwon, Ie B. Park, Kyu J. Ahn, Cheol‐Young Park, Su K. Kwon, Hye S. Kim, Seok W. Park, Sin G. Kim, Min K. Moon, Eun S. Kim, Choon H. Chung, Kang S. Park, Mikyung Kim, Dong J. Chung, Chang B. Lee, Tae H. Kim, Moon‐Kyu Lee
Diabetes, Obesity and Metabolism.2020; 22(9): 1527. CrossRef Effect of Switching from Linagliptin to Teneligliptin Dipeptidyl Peptidase-4 Inhibitors in Older Patients with Type 2 Diabetes Mellitus
Eugene Han, Minyoung Lee, Yong-ho Lee, Hye Soon Kim, Byung-wan Lee, Bong-Soo Cha, Eun Seok Kang
Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy.2020; Volume 13: 4113. CrossRef- Efficacy and safety of novel dipeptidyl-peptidase-4 inhibitor evogliptin in the management of type 2 diabetes mellitus: A meta-analysis
Deep Dutta, Saptarshi Bhattacharya, Aishwarya Krishnamurthy, LokeshKumar Sharma, Meha Sharma
Indian Journal of Endocrinology and Metabolism.2020; 24(5): 434. CrossRef
- Targeting cluster of differentiation 26 / dipeptidyl peptidase 4 (CD26/DPP4) in organ fibrosis
- Complications
- Gemigliptin Attenuates Renal Fibrosis Through Down-Regulation of the NLRP3 Inflammasome
- Jung Beom Seo, Yeon-Kyung Choi, Hye-In Woo, Yun-A Jung, Sungwoo Lee, Seunghyeong Lee, Mihyang Park, In-Kyu Lee, Gwon-Soo Jung, Keun-Gyu Park
- Diabetes Metab J. 2019;43(6):830-839. Published online March 5, 2019
- DOI: https://doi.org/10.4093/dmj.2018.0181
- 5,422 View
- 128 Download
- 23 Web of Science
- 24 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background The hypoglycemic drugs dipeptidyl peptidase-4 (DPP-4) inhibitors have proven protective effects on diabetic kidney disease, including renal fibrosis. Although NOD-like receptor protein 3 (NLRP3) inflammasome activation is known to play an important role in the progression of renal fibrosis, the impact of DPP-4 inhibition on NLRP3-mediated inflammation while ameliorating renal fibrosis has not been fully elucidated. Here, we report that the renoprotective effect of gemigliptin is associated with a reduction in NLRP3-mediated inflammation in a murine model of renal fibrosis.
Methods We examined the effects of gemigliptin on renal tubulointerstitial fibrosis induced in mice by unilateral ureteral obstruction (UUO). Using immunohistochemical and Western blot analysis, we quantitated components of the NLRP3 inflammasome in kidneys with and without gemigliptin treatment, and
in vitro in human kidney tubular epithelial human renal proximal tubule cells (HK-2) cells, we further analyzed the effect of gemigliptin on transforming growth factor-β (TGF-β)-stimulated production of profibrotic proteins.Results Immunohistological examination revealed that gemigliptin ameliorated UUO-induced tubular atrophy and renal fibrosis. Gemigliptin-treated kidneys showed a reduction in levels of NLRP3, apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC), caspase-1, and interleukin-1β, which had all been markedly increased by UUO. In line with the
in vivo Conclusion The present study shows that activation of the NLRP3 inflammasome contributes to UUO-induced renal fibrosis and the renoprotective effect of gemigliptin is associated with attenuation of NLRP3 inflammasome activation.
-
Citations
Citations to this article as recorded by- Novel pharmacological interventions for diabetic kidney disease
Seng Kiong Tan, Jairo A. Pinzon-Cortes, Mark E. Cooper
Current Opinion in Nephrology & Hypertension.2024; 33(1): 13. CrossRef - Integrated analysis reveals crosstalk between pyroptosis and immune regulation in renal fibrosis
Fengxia Bai, Longchao Han, Jifeng Yang, Yuxiu Liu, Xiangmeng Li, Yaqin Wang, Ruijian Jiang, Zhaomu Zeng, Yan Gao, Haisong Zhang
Frontiers in Immunology.2024;[Epub] CrossRef - Di (2-ethylhexyl) phthalate and polystyrene microplastics co-exposure caused oxidative stress to activate NF-κB/NLRP3 pathway aggravated pyroptosis and inflammation in mouse kidney
Shanshan Li, Xuedie Gu, Muyue Zhang, Qihang Jiang, Tong Xu
Science of The Total Environment.2024; 926: 171817. CrossRef - Fluorofenidone attenuates renal fibrosis by inhibiting lysosomal cathepsin‑mediated NLRP3 inflammasome activation
Linfeng Zheng, Wenjuan Mei, Jing Zhou, Xin Wei, Zhijuan Huang, Xiaozhen Lin, Li Zhang, Wei Liu, Qian Wu, Jinhong Li, Yan Yan
Experimental and Therapeutic Medicine.2024;[Epub] CrossRef - HIF1α-BNIP3-mediated mitophagy protects against renal fibrosis by decreasing ROS and inhibiting activation of the NLRP3 inflammasome
Jialin Li, Qisheng Lin, Xinghua Shao, Shu Li, Xuying Zhu, Jingkui Wu, Shan Mou, Leyi Gu, Qin Wang, Minfang Zhang, Kaiqi Zhang, Jiayue Lu, Zhaohui Ni
Cell Death & Disease.2023;[Epub] CrossRef - Pyroptosis in renal inflammation and fibrosis: current knowledge and clinical significance
Ya Liu, Haibo Lei, Wenyou Zhang, Qichang Xing, Renzhu Liu, Shiwei Wu, Zheng Liu, Qingzi Yan, Wencan Li, Xiang Liu, Yixiang Hu
Cell Death & Disease.2023;[Epub] CrossRef - Tubular injury in diabetic kidney disease: molecular mechanisms and potential therapeutic perspectives
Yu Wang, Mingyue Jin, Chak Kwong Cheng, Qiang Li
Frontiers in Endocrinology.2023;[Epub] CrossRef - Hederagenin inhibits high glucose‐induced fibrosis in human renal cells by suppression of NLRP3 inflammasome activation through reducing cathepsin B expression
Guohua Yang, Wang Yang, Hairong Jiang, Qing Yi, Wei Ma
Chemical Biology & Drug Design.2023; 102(6): 1409. CrossRef - Obstructive nephropathy and molecular pathophysiology of renal interstitial fibrosis
Rikke Nørregaard, Henricus A. M. Mutsaers, Jørgen Frøkiær, Tae-Hwan Kwon
Physiological Reviews.2023; 103(4): 2847. CrossRef - Adenine model of chronic renal failure in rats to determine whether MCC950, an NLRP3 inflammasome inhibitor, is a renopreventive
Mahmoud S. Sabra, Fahmy K. Hemida, Essmat A. H. Allam
BMC Nephrology.2023;[Epub] CrossRef - Gut microbiota dysbiosis promotes age-related atrial fibrillation by lipopolysaccharide and glucose-induced activation of NLRP3-inflammasome
Yun Zhang, Song Zhang, Bolin Li, Yingchun Luo, Yongtai Gong, Xuexin Jin, Jiawei Zhang, Yun Zhou, Xiaozhen Zhuo, Zixi Wang, Xinbo Zhao, Xuejie Han, Yunlong Gao, Hui Yu, Desen Liang, Shiqi Zhao, Danghui Sun, Dingyu Wang, Wei Xu, Guangjin Qu, Wanlan Bo, Dan
Cardiovascular Research.2022; 118(3): 785. CrossRef - The NLRP3 inflammasome in fibrosis and aging: The known unknowns
Yanqing Liu, Xuezeng Xu, Wangrui Lei, Yuxuan Hou, Yan Zhang, Ran Tang, Zhi Yang, Ye Tian, Yanli Zhu, Changyu Wang, Chao Deng, Shaofei Zhang, Yang Yang
Ageing Research Reviews.2022; 79: 101638. CrossRef - Research progress of endothelial‐mesenchymal transition in diabetic kidney disease
Ying Chen, Hang Zou, Hongwei Lu, Hong Xiang, Shuhua Chen
Journal of Cellular and Molecular Medicine.2022; 26(12): 3313. CrossRef - Exploring the mechanism of Shendi Bushen capsule in anti-renal fibrosis using metabolomics theory and network analysis
Tianwei Meng, Hong Chang, Hongyu Meng
Molecular Omics.2022; 18(9): 873. CrossRef - Gemigliptin suppresses salivary dysfunction in streptozotocin-induced diabetic rats
Wan Seok Kang, Woo Kwon Jung, Su-Bin Park, Hyung Rae Kim, Junghyun Kim
Biomedicine & Pharmacotherapy.2021; 137: 111297. CrossRef - Long‐Term Dipeptidyl Peptidase 4 Inhibition Worsens Hypertension and Renal and Cardiac Abnormalities in Obese Spontaneously Hypertensive Heart Failure Rats
Edwin K. Jackson, Zaichuan Mi, Delbert G. Gillespie, Dongmei Cheng, Stevan P. Tofovic
Journal of the American Heart Association.2021;[Epub] CrossRef - Disulfiram inhibits inflammation and fibrosis in a rat unilateral ureteral obstruction model by inhibiting gasdermin D cleavage and pyroptosis
Yu Zhang, Ruicheng Zhang, Xiaohu Han
Inflammation Research.2021; 70(5): 543. CrossRef - Inflammasome as an Effective Platform for Fibrosis Therapy
Ting-Ting Chen, Feng Xiao, Nan Li, Shan Shan, Meng Qi, Zi-Ying Wang, Sheng-Nan Zhang, Wei Wei, Wu-Yi Sun
Journal of Inflammation Research.2021; Volume 14: 1575. CrossRef - Targeting Dermal Fibroblast Subtypes in Antifibrotic Therapy: Surface Marker as a Cellular Identity or a Functional Entity?
Xin Huang, Yimin Khoong, Chengyao Han, Dai Su, Hao Ma, Shuchen Gu, Qingfeng Li, Tao Zan
Frontiers in Physiology.2021;[Epub] CrossRef - Linagliptin Protects against Endotoxin-Induced Acute Kidney Injury in Rats by Decreasing Inflammatory Cytokines and Reactive Oxygen Species
Tsung-Jui Wu, Yi-Jen Hsieh, Chia-Wen Lu, Chung-Jen Lee, Bang-Gee Hsu
International Journal of Molecular Sciences.2021; 22(20): 11190. CrossRef - Psidium guajava Flavonoids Prevent NLRP3 Inflammasome Activation and Alleviate the Pancreatic Fibrosis in a Chronic Pancreatitis Mouse Model
Guixian Zhang, Liming Tang, Hongbin Liu, Dawei Liu, Manxue Wang, Jun Cai, Weijun Liu, Wei Nie, Yi Zhang, Xiaomeng Yu
The American Journal of Chinese Medicine.2021; 49(08): 2001. CrossRef - Effect and Regulation of the NLRP3 Inflammasome During Renal Fibrosis
Hong Zhang, Zhengchao Wang
Frontiers in Cell and Developmental Biology.2020;[Epub] CrossRef - Zhen-Wu-Tang Protects IgA Nephropathy in Rats by Regulating Exosomes to Inhibit NF-κB/NLRP3 Pathway
Honglian Li, Ruirui Lu, Yu Pang, Jicheng Li, Yiwen Cao, Hongxin Fu, Guoxing Fang, Qiuhe Chen, Bihao Liu, Junbiao Wu, Yuan Zhou, Jiuyao Zhou
Frontiers in Pharmacology.2020;[Epub] CrossRef - Protective effect of exogenous hydrogen sulfide on diaphragm muscle fibrosis in streptozotocin-induced diabetic rats
Rui Yang, Qiang Jia, Yan Li, Shomaila Mehmood
Experimental Biology and Medicine.2020; 245(14): 1280. CrossRef
- Novel pharmacological interventions for diabetic kidney disease
- Complications
- Serum Levels of PCSK9 Are Associated with Coronary Angiographic Severity in Patients with Acute Coronary Syndrome
- Kwi-Hyun Bae, Sung Woo Kim, Yeon-Kyung Choi, Jung Beom Seo, Namkyun Kim, Chang-Yeon Kim, Won Kee Lee, Sungwoo Lee, Jung Guk Kim, In-Kyu Lee, Jang Hoon Lee, Keun-Gyu Park
- Diabetes Metab J. 2018;42(3):207-214. Published online May 2, 2018
- DOI: https://doi.org/10.4093/dmj.2017.0081
- 4,937 View
- 54 Download
- 25 Web of Science
- 23 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background Proprotein convertase subtilisin/kexin type 9 (PCSK9) is a circulating protein that promotes degradation of the low density lipoprotein receptor. PCSK9 has emerged as a target for lipid-lowering therapy, but the predictive value of the serum level of PCSK9 for the severity of coronary disease is largely unknown.
Methods From December 2009 to July 2012, 121 individuals who underwent coronary angiography (CAG) because of clinically suspected acute coronary syndrome were enrolled in this study. Serum levels of PCSK9 and metabolic parameters were measured. SYNTAX (SYNergy between percutaneous coronary intervention with [paclitaxel-eluting] TAXUS stent and cardiac surgery) and GRACE (Global Registry of Acute Coronary Events) scores were calculated.
Results Individuals with CAG lesions (
n =100) had significantly higher levels of PCSK9 than those without lesions (n =21). The study population was stratified into three groups according to serum levels of PCSK9. The odds radio for occurrence of one or more CAG lesions was significantly higher in the group with the highest level of PCSK9 (odds ratio, 7.468;P =0.011) than in the group with the lowest level of PCSK9. Serum PCSK9 was positively associated with the number of involved coronary arteries. Multivariable linear regression indicated that levels of PCSK9 were positively correlated with GRACE risk scores and SYNTAX scores.Conclusion Serum PCSK9 concentrations are higher in patients with coronary artery lesions, and are associated with SYNTAX and GRACE scores, suggesting that PCSK9 is a potential biomarker of the severity of coronary artery disease.
-
Citations
Citations to this article as recorded by- Clinical Significance of PCSK9 and Soluble P-selectin in Predicting Major Adverse Cardiovascular Events After Primary Percutaneous Coronary Intervention in Patients with Acute Coronary Syndrome
Yao Yao, Qining Qiu, Xiaoye Li, Zi Wang, Shikun Xu, Qianzhou Lv
Cardiovascular Innovations and Applications.2024;[Epub] CrossRef - Stratification in Heterozygous Familial Hypercholesterolemia: Imaging, Biomarkers, and Genetic Testing
Pablo Corral, Carlos A. Aguilar Salinas, María Gabriela Matta, Valeria Zago, Laura Schreier
Current Atherosclerosis Reports.2023; 25(12): 899. CrossRef - Relationship of sortilin and proprotein convertase subtilisin/kexin type 9 in blood serum with the severity of carotid and coronary atherosclerosis in hypertensive patients
Yu. Yu. Vukolova, I. V. Gubareva
Russian Journal of Cardiology.2022; 27(2S): 4903. CrossRef - PCSK9 Inhibition could be Effective for Acute Myocardial Infarction
Baris Gencer, François Mach
Current Medicinal Chemistry.2022; 29(6): 1016. CrossRef - Peri-event plasma PCSK9 and hsCRP after an acute myocardial infarction correlate with early deterioration of left ventricular ejection fraction: a cohort study
Lina S. Silva-Bermúdez, Andrea Vargas-Villanueva, Carlos A. Sánchez-Vallejo, Ana C. Palacio, Andrés F. Buitrago, Carlos O. Mendivil
Lipids in Health and Disease.2022;[Epub] CrossRef - Plasma Levels of Proprotein Convertase Subtilisin/Kexin Type 9 Are Inversely Associated with N-Terminal Pro B-Type Natriuretic Peptide in Older Men and Women
Francesco Spannella, Federico Giulietti, Roberta Galeazzi, Anna Passarelli, Serena Re, Chiara Di Pentima, Massimiliano Allevi, Paolo Magni, Riccardo Sarzani
Biomedicines.2022; 10(8): 1961. CrossRef - Sex difference in circulating PCSK9 and its clinical implications
Fang Jia, Si-Fan Fei, De-Bing Tong, Cong Xue, Jian-Jun Li
Frontiers in Pharmacology.2022;[Epub] CrossRef - Physiology and role of PCSK9 in vascular disease: Potential impact of localized PCSK9 in vascular wall
Yanan Guo, Binjie Yan, Yu Gui, Zhihan Tang, Shi Tai, Shenghua Zhou, Xi‐Long Zheng
Journal of Cellular Physiology.2021; 236(4): 2333. CrossRef - PCSK9 levels do not predict severity and recurrence of cardiovascular events in patients with acute myocardial infarction
Marianne Zeller, Gilles Lambert, Michel Farnier, Maud Maza, Brice Nativel, Luc Rochette, Catherine Vergely, Yves Cottin
Nutrition, Metabolism and Cardiovascular Diseases.2021; 31(3): 880. CrossRef - Pcsk9 is associated with severity of coronary artery lesions in male patients with premature myocardial infarction
Jing Gao, Ya-Nan Yang, Zhuang Cui, Si-Yuan Feng, Jing Ma, Chang-Ping Li, Yin Liu
Lipids in Health and Disease.2021;[Epub] CrossRef - Circulating Biomarkers for Cardiovascular Disease Risk Prediction in Patients With Cardiovascular Disease
Yuen-Kwun Wong, Hung-Fat Tse
Frontiers in Cardiovascular Medicine.2021;[Epub] CrossRef - Association Between Circulating Proprotein Convertase Subtilisin/Kexin Type 9 Concentrations and Cardiovascular Events in Cardiovascular Disease: A Systemic Review and Meta-Analysis
Jiahui Liu, Fangfang Fan, Xingyu Luo, Wenjun Ji, Yaokun Liu, Yan Zhang, Bo Zheng
Frontiers in Cardiovascular Medicine.2021;[Epub] CrossRef - PCSK9 in acute coronary syndrome: analysis of associations with clinical and laboratory characteristics
Yu. A. Drenina, K. Yu. Nikolaev
Cardiovascular Therapy and Prevention.2020; 19(4): 2484. CrossRef - Relation of Proprotein Convertase Subtilisin/Kexin Type 9 to Cardiovascular Outcomes in Patients Undergoing Percutaneous Coronary Intervention
Ik Jun Choi, Sungmin Lim, Dongjae Lee, Won Jik Lee, Kwan Yong Lee, Mi-Jeong Kim, Doo Soo Jeon
The American Journal of Cardiology.2020; 133: 54. CrossRef - A Narrative Review and Expert Panel Recommendations on Dyslipidaemia Management After Acute Coronary Syndrome in Countries Outside Western Europe and North America
Ashraf Reda, Wael Almahmeed, Idit Dobrecky-Mery, Po-Hsun Huang, Ursulo Juarez-Herrera, Naresh Ranjith, Tobias Sayre, Miguel Urina-Triana
Advances in Therapy.2020; 37(5): 1754. CrossRef - PCSK9 concentrations in different stages of subclinical atherosclerosis and their relationship with inflammation
Štefan Tóth, Peter Olexa, Zdenka Hertelyová, Peter Štefanič, Ivan Kopolovets, Peter Berek, Vladimir Filip, Ryan Chakravarty, Monika Široká, Daniel Pella
Open Chemistry.2020; 18(1): 1011. CrossRef - Association of serum proprotein convertase subtilisin/kexin type 9 with early atherosclerosis in newly diagnosed type 2 diabetes mellitus
Wen Guo, Yingyun Gong, Jie Li, Pei Qin, Jing Lu, Xiaona Li, Wenfang Zhu, Nianzhen Xu, Hongwen Zhou, Qun Zhang
Nutrition, Metabolism and Cardiovascular Diseases.2019; 29(8): 815. CrossRef - PCSK9 and atherosclerosis burden in the coronary arteries of patients undergoing coronary angiography
Yunes Panahi, Mohsen Sadeghi Ghahrodi, Mohsen Jamshir, Mohammad Amin Safarpour, Vanessa Bianconi, Matteo Pirro, Maryam Moshkani Farahani, Amirhossein Sahebkar
Clinical Biochemistry.2019; 74: 12. CrossRef - Association of PCSK9 plasma levels with metabolic patterns and coronary atherosclerosis in patients with stable angina
Chiara Caselli, Serena Del Turco, Rosetta Ragusa, Valentina Lorenzoni, Michiel De Graaf, Giuseppina Basta, Arthur Scholte, Raffaele De Caterina, Danilo Neglia
Cardiovascular Diabetology.2019;[Epub] CrossRef - PCSK9 inhibition and inflammation: A narrative review
Massimiliano Ruscica, Lale Tokgözoğlu, Alberto Corsini, Cesare R. Sirtori
Atherosclerosis.2019; 288: 146. CrossRef - Letter: Serum Levels of PCSK9 Are Associated with Coronary Angiographic Severity in Patients with Acute Coronary Syndrome (Diabetes Metab J 2018;42:207-14)
Jin Hwa Kim
Diabetes & Metabolism Journal.2018; 42(4): 348. CrossRef - Response: Serum Levels of PCSK9 Are Associated with Coronary Angiographic Severity in Patients with Acute Coronary Syndrome (Diabetes Metab J 2018;42:207-14)
Sung Woo Kim, Keun-Gyu Park
Diabetes & Metabolism Journal.2018; 42(4): 350. CrossRef - Serum PCSK9 levels, but not PCSK9 polymorphisms, are associated with CAD risk and lipid profiles in southern Chinese Han population
Gaojun Cai, Lei Yu, Zhiying Huang, Li Li, Xingli Fu
Lipids in Health and Disease.2018;[Epub] CrossRef
- Clinical Significance of PCSK9 and Soluble P-selectin in Predicting Major Adverse Cardiovascular Events After Primary Percutaneous Coronary Intervention in Patients with Acute Coronary Syndrome

 KDA
KDA
 First
First Prev
Prev





